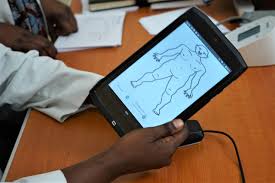
Uganda has taken a big step in protecting patients from harmful drug side effects by introducing a simple mobile app called Med Safety.
The free smartphone tool is linked to the country’s National Drug Authority (NDA) and is already changing how medicine safety is monitored.
According to a new study published in The Lancet Global Health on September 18, 2025, the app has led to a 73% increase in reporting of drug side effects, and a 92% increase in reports related to dolutegravir, a widely used HIV treatment.
For years, Uganda—like many low- and middle-income countries—struggled with under-reporting of adverse drug reactions (ADRs). Patients often experienced side effects such as rashes, dizziness, weight changes, or fatigue, but these cases were rarely captured in official records.
This left regulators without enough evidence to take action, putting millions of patients—especially those on lifelong HIV treatment—at risk.
Traditionally, health workers had to fill out long paper forms or call supervisors to report suspected side effects. With Med Safety, they can now record symptoms on their phones and upload reports instantly to the NDA.
Nurses like Sarah Nabirye, who works in a rural clinic in central Uganda, say the app has made a huge difference. “What used to take hours of paperwork can now be reported in minutes,” she explained.
The trial was the largest of its kind ever conducted in Africa, covering 367 health facilities and involving over 2,400 healthcare workers.
Researchers compared traditional reporting methods with the mobile app and found that Med Safety captured both mild and severe side effects, giving health officials a much clearer picture of how medicines affect patients in real life.
In total, 3,634 suspected drug reactions were reported during the study—over a third of them classified as serious.
These included reactions not only from HIV drugs but also from medicines used to treat tuberculosis, malaria, high blood pressure, diabetes, and common infections.
The project was led by Makerere University in partnership with the University of Liverpool, Uganda’s National Drug Authority, the UK Medicines and Healthcare products Regulatory Agency, the Uganda AIDS Control Programme, and the African Union Development Agency.
Dr. Ronald Kiguba, the lead researcher, said:
“The trial shows that digital tools like Med Safety can transform drug safety monitoring in real-world settings. Scaling this up will help Uganda and other countries make better, evidence-based decisions to protect patients.”
With millions of Ugandans relying on daily medication, especially for HIV, malaria, and chronic conditions, the adoption of Med Safety could save lives and restore confidence in treatment programs.
Officials are now exploring how to roll out the app nationwide and extend it to other African countries facing similar challenges.