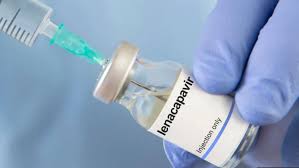
The Kenyan government has taken a major step in the fight against HIV by recommending the registration of Lenacapavir, a new long-acting HIV prevention drug.
In a statement released on January 9, 2026, the Ministry of Health, through the Pharmacy and Poisons Board, announced that it has approved the recommendation for Lenacapavir 300 mg tablets and Lenacapavir 464 mg injectable solution. The drug will be used for HIV pre-exposure prophylaxis (PrEP), which helps prevent HIV infection among people who are at high risk.
Health Cabinet Secretary Aden Duale said the decision followed a thorough scientific review to confirm the drug’s safety, quality, and effectiveness, in line with Kenyan law and international standards. Lenacapavir has also received approval from the World Health Organization (WHO) for HIV prevention.
Unlike the current daily oral PrEP pills, Lenacapavir is a long-acting injectable that is taken only twice a year. This makes it a strong option for people who struggle with taking daily medication due to pill fatigue, stigma, or difficulty sticking to a routine.
“The long-acting nature of Lenacapavir offers a powerful alternative to daily pills and will help more people protect themselves from HIV,” said CS Duale.
Kenya is now among the first African countries to recommend Lenacapavir for registration. The government says this move shows Kenya’s leadership in adopting new health technologies and aligns with global HIV prevention guidelines.
The Ministry of Health noted that while daily PrEP has been rolled out nationwide, some users face challenges with long-term daily use. Introducing long-acting prevention options like Lenacapavir is expected to increase access, improve adherence, and reduce new HIV infections.
Duale added that Kenya has been prioritised for the initial rollout through international partnerships, with plans in place to ensure fair access for communities most at risk.
The ministry reaffirmed its commitment to ending HIV as a public health threat, saying all approved medicines will continue to meet strict safety and regulatory standards.