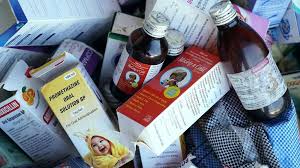
The World Health Organization (WHO) has issued an urgent global alert warning about three contaminated cough syrups discovered in India, which have been linked to the deaths of several young children.
The contaminated medicines—Coldrif from Sresan Pharmaceutical, Respifresh TR from Rednex Pharmaceuticals, and ReLife from Shape Pharma—were found to contain diethylene glycol, a highly toxic chemical often used in industrial products like antifreeze.
According to WHO, the levels of diethylene glycol detected were nearly 500 times higher than the safe limit, making the syrups extremely dangerous if consumed.
The organization has urged all countries to immediately check for the presence of these products in their markets and to report any findings to health authorities.
India’s Central Drugs Standard Control Organization (CDSCO) reported that the contaminated syrups were consumed by children under the age of five in the Chhindwara district of Madhya Pradesh, leading to multiple deaths.
Investigations revealed that the children had been given the cough syrups to treat common colds and coughs before developing severe complications, including kidney failure—a common symptom of diethylene glycol poisoning.
Indian authorities have assured the WHO that there is no evidence the contaminated syrups were exported to other countries. The U.S.
Food and Drug Administration (FDA) also confirmed that none of the affected batches had been imported into the United States.
However, the WHO warned that contaminated or counterfeit medicines can easily cross borders, especially through unregulated supply chains and online markets.
The organization called on all governments to strengthen drug quality surveillance and ensure that only approved, safe pharmaceuticals are available to consumers.
This latest warning comes after several incidents in recent years where contaminated medicines from India and other countries caused child deaths.
In 2022, toxic cough syrups linked to manufacturers in India and Indonesia were blamed for more than 300 child deaths in Gambia, Uzbekistan, and Cameroon, prompting global outrage and tighter quality controls.
In its statement, WHO said: “These products pose serious health risks, including death, particularly in children. Countries must take urgent action to detect and remove these contaminated syrups from circulation.”
Parents are advised to avoid buying medicines from unverified sources and to always check packaging for batch numbers, manufacturing details, and expiry dates.
The organization also reminded health professionals to report any adverse drug reactions immediately to national drug safety authorities.